Watershed provides a controlled humidity environment for a wide range of common sample preparation tasks, including
- separating crystals from each other, from precipitate, and from protein skins that may form over crystallization drops;
- soaking crystals in solutions containing cryoprotectants, heavy atoms or ligands;
- harvesting crystals from drops onto X-ray sample mounts;
- blotting or wicking away excess liquid from crystals to reduce background X-ray scatter and increase sample cooling rates during plunge cooling;
- placing crystals in MiTeGen’s MicroRT capillaries or glass capillaries for room temperature data collection; and
- adjusting the hydration of crystals to a desired relative humidity, which may be different from their as-grown relative humidity, to modify protein structure or optimize diffraction properties.
Watershed consists of a water tank, a control unit, and two workstations that provide controllable relative humidities between 50% and >90% RH.
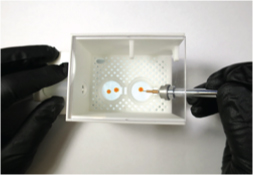
The Humidified Chamber Workstation accepts drops on 18 or 22 mm cover slips or on standard microscope slides. Crystals can be harvested and manipulated within the workstation using standard crystal loops, goniometer bases, and magnetic wands, while being observed from above using a long-working distance stereo microscope. Once mounted, the sample can be transferred to a cryovial containing humid air, and the sample + cryovial can then be removed and transported to, e.g., a cryocooling setup.
The Directed Nozzle Workstation can be used with crystals in multiwell crystallization plates to provide a humidified air flow directed at a well being examined. This workstation can also be used with a laboratory X-ray source to controllably vary the hydration of a crystal during diffraction data collection.
Read the MiTeGen Watershed User Manual to learn more
Maintaining and controlling sample hydration is a critical problem in biomolecular crystallography, as well as in cell biology, assisted reproduction, and related fields. Watershed provides a convenient, robust, low-cost solution to this problem.
When manipulating crystals contained in liquid drops on cover slips, glass slides, or in multiple well microplates, evaporation and protein degradation can lead to formation of precipitate and protein skins, to crystal aggregation and adhesion, and to crystal cracking and other damage.
Evaporation limits the time that a given drop can be exposed to air during crystal manipulations and harvesting before the drop and remaining crystals within it become unusable.
Evaporation during crystal soaks in solutions containing cryoprotectants, heavy atom compounds, or ligands can lead to uncertainty and to crystal-to-crystal variations in the concentrations of these solutes within the crystal.
Evaporation during these steps and during crystal harvesting onto a crystallography loop or mount can cause degradation in crystal diffraction properties and crystal-to-crystal variations in unit cell dimensions and protein structure.
Crystal dehydration during post-growth handling is the overwhelmingly dominant cause of crystal nonisomorphism in room temperature X-ray data collection, and an important cause of cryogenic temperature nonisomorphism. Typical room-temperature unit cell volume variations of 1% or more decrease to less than 0.2% when crystal hydration is carefully controlled.
On the other hand, modest controlled dehydration can, for perhaps 5-10% of protein crystals, cause improvements in overall crystal order and diffraction properties, and provides a useful salvage approach when crystal diffraction is inadequate.
Watershed provides a convenient, low cost solution for controlling the hydration environment during crystal harvesting, manipulations, mounting, and X-ray data collection.
By using Watershed,
- Drop and sample working times can be increased by a factor of 10 or more relative to typical laboratory environments having relative humidities below 50%.
- More crystals can be harvested from each drop before it becomes unusable.
- Crystal isomorphism is improved, improving data quality and sometimes reducing the number of crystals required for structure determination.
- Perturbations of protein structures away from their biologically relevant forms are reduced.
- Quality and reproducibility of data are improved.
Watershed mixes high-humidity, room-temperature air (generated by bubbling dry air through room temperature water) with dry air to obtain an air stream with a desired relative humidity.
Relative Humidity Range: 72% to >90% RH
Water content of the compressed air supply affects the lowest achievable humidity. 72% RH tested with 23°C water and 6% RH air source.
Water tank capacity: 2.5 gallons / 10 liters
Requirements:
Power source: 110-125 VAC, 50-60 Hz, preferably with GFCI protection
Source of dry air or nitrogen at a pressure of 25 psi
Distilled or deionized water
Control unit:
Dimensions: 5.3 (H) x 14.0 (W) x 10.0 (D) inches / 135 x 357 x 255 mm
Weight: 11.5 lbs / 5.2 kg
Water Tank:
Dimensions: 10.2 (H) x 14.0 (W) x 9.3 (D) inches / 260 x 357 x 235 mm
Weight: 5.0 lbs / 2.3 kg empty; 26 lbs / 11.8 kg full
Humidified Enclosure Workstation:
Dimensions: 2.6 (H) x 4.5 (W) x 2.2 (D) inches / 66 x 115 x 55 mm
Accepts two 18 mm or 22 mm round cover slips or one 25 x 75 mm microscope slide
Required microscope working distance: 1.8 inches / 45 mm minimum
Positionable Nozzle Workstation:
Magnetic base diameter: 2 inches / 54 mm
Positionable tubing length: 19 inches / 500 mm
“Improved reproducibility of unit-cell parameters in macromolecular cryocrystallography by limiting dehydration during crystal mounting,” Christopher Farley, Geoffry Burks, Thomas Siegert and Douglas H. Juers, Acta Cryst. D70, 2111-2124 (2014).
“A generic protocol for protein crystal dehydration using the HC1b humidity controller,” Carina M. C. Lobley, James Sandy, Juan Sanchez-Weatherby, Marco Mazzorana, Tobias Krojer, Radosław P. Nowak, and Thomas L. Sorensen, Acta Cryst. D72, 629-640 (2016).
“Automation and Experience of Controlled Crystal Dehydration: Results from the European Synchrotron HC1 Collaboration,” Matthew W. Bowler, Uwe Mueller, Manfred S. Weiss, Juan Sanchez-Weatherby, Thomas L-M. Sorensen, Marjolein M. G. M. Thunnissen,, Thomas Ursby, Alexandre Gobbo,,Silvia Russi, Michael G. Bowler, Sandor Brockhauser, Olof Svensson, and Florent Cipriani, Crystal Growth and Design 15, 1043-1054 (2015).
“Humidity control and hydrophilic glue coating applied to mounted protein crystals improves X-ray diffraction experiments,” Seiki Baba, Takeshi Hoshino, Len Ito and Takashi Kumasaka, Acta Cryst. D69, 1839-1849 (2013).
“Measurement of the equilibrium relative humidity for common precipitant concentrations: facilitating controlled dehydration experiments,” by M. J. Wheeler, S. Russi, M. G. Bowler, and M. W. Bowler, Acta Cryst. F 68, 111-114, (2011).
“Improving diffraction by humidity control: a novel device compatible with X-ray beamlines.” Juan Sanchez-Weatherby, Matthew W. Bowler, Julien Huet, Alexandre Gobbo, Franck Felisaz, Bernard Lavault, Raphael Moya, Jan Kadlec, Raimond B. G. Ravelli, and Florent Cipriani, Acta Cryst. D65, 1237-1246 (2009).
“A novel free-mounting system for protein crystals: transformation and improvement of diffraction power by accurately controlled humidity changes,” Reiner Kiefersauer, Manuel E. Than, Holger Dobbek, Lothar Gremer, Marcos Melero, Stefan Strobl, Joao M. Dieas, Twefik Soulimane, and Robert Huber, J. Appl. Cryst. 33, 1223-1230 (2000).
“A design of crystal mounting cell that allows the controlled variation of humidity at the protein crystal during X-ray diffraction,” M. G. Pickford, E. F. Garman, E. Y. Jones, and D. I. Stuart, J.Appl. Cryst. 26, 465-466 (1993).









 Custom kits developed for crystallographers in cooperation with Carl Zeiss. All MiTeGen Kits from Zeiss…
Custom kits developed for crystallographers in cooperation with Carl Zeiss. All MiTeGen Kits from Zeiss… JBScreen JCSG++ is a sparse matrix screen optimized for initial screening of crystallization conditions of…
JBScreen JCSG++ is a sparse matrix screen optimized for initial screening of crystallization conditions of… MiTeGen’s Small Crystal Harvesting Kit™ has everything you need to mount and collect X-ray data from…
MiTeGen’s Small Crystal Harvesting Kit™ has everything you need to mount and collect X-ray data from…


